Calculate The Ph Of The Following 0 5 M M Hcl If Chegg Hot Sex Picture
Calculate Ph of 105M of naoh Brainly.in

So the negative log of 5.6 times 10 to the negative 10. Is going to give us a pKa value of 9.25 when we round. So pKa is equal to 9.25. So we're gonna plug that into our Henderson-Hasselbalch equation right here. So the pH of our buffer solution is equal to 9.25 plus the log of the concentration of A minus, our base.
[Solved] Calculate the pH of the solution resulting from the addition of... Course Hero
A solution of a strong alkali at concentration 1 M (1 mol/L) has a pH of 14. Thus, in most problems that arise pH values lie mostly in the range 0 to 14, though negative pH values and values above 14 are entirely possible. Weak acid/base. Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a bit more complicated.
SOLVEDA plot of pH vs volume of 0.05 M NaOH solution was obtained for titrating 0.4055 g of a
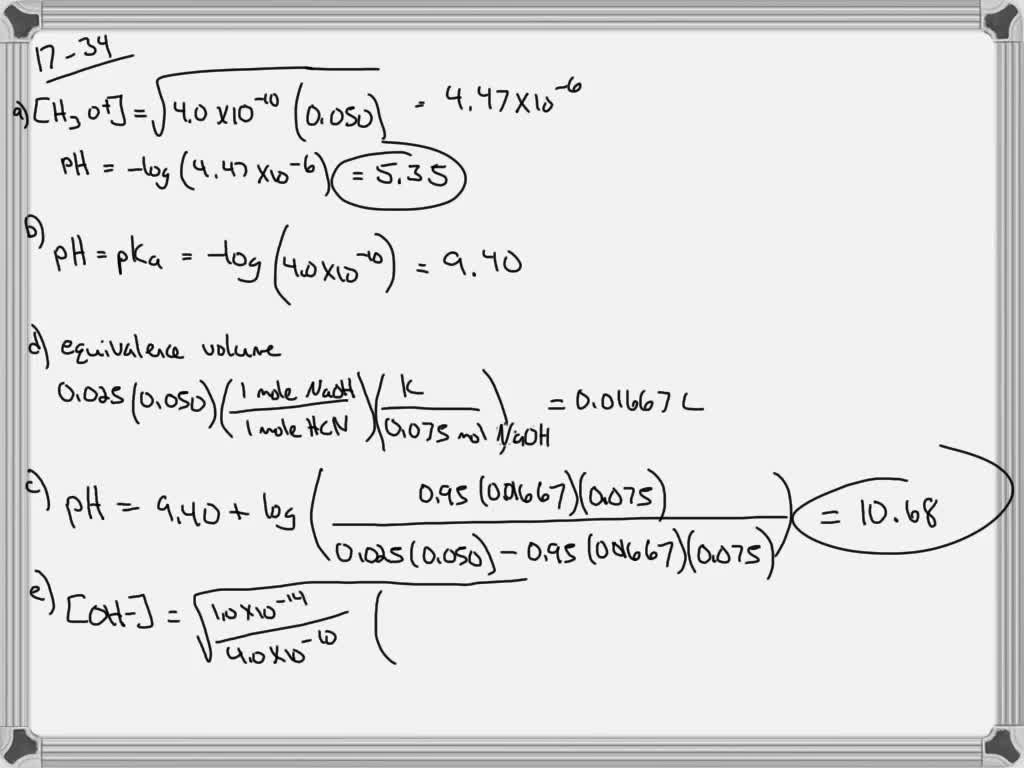
Compute pH of the 0.1 M solution of acetic acid (pKa=4.76). Solve example 1. Example 2 What is the pH of the 10-7 M HCl? Solve example 2. Example 3 Calculate pH and pOH of the solution containing 0.1M of H3PO4 (pKa1=2.12, pKa2=7.21, pKa3=12.67)? Solve example 3. Example 4 What is pH of the solution obtained by mixing 10 ml 0.5 M of C6H5COONa.
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube
NaOH is a strong base, so this will produce 0.1mol/L of OH ions in solution. This will produce a pH of 13. You will need to take the negative log of 0.1 to find the pOH. This will work out to be 1. Since pH + pOH = 14. We can calculate the pH to be 13. This assumption we made about the base can only be used for strong bases which dissociate.
0.5 M NaOH Solution YouTube

pH + pOH = 14 (Eq. 3) This relationship can be used to convert between pH and pOH . In combination with Eq. 1a/b and Eq. 2a/b, we can always relate pOH and/or pH to [ OH −] and [ H +] . For a derivation of this equation, check out the article on the autoionization of water.
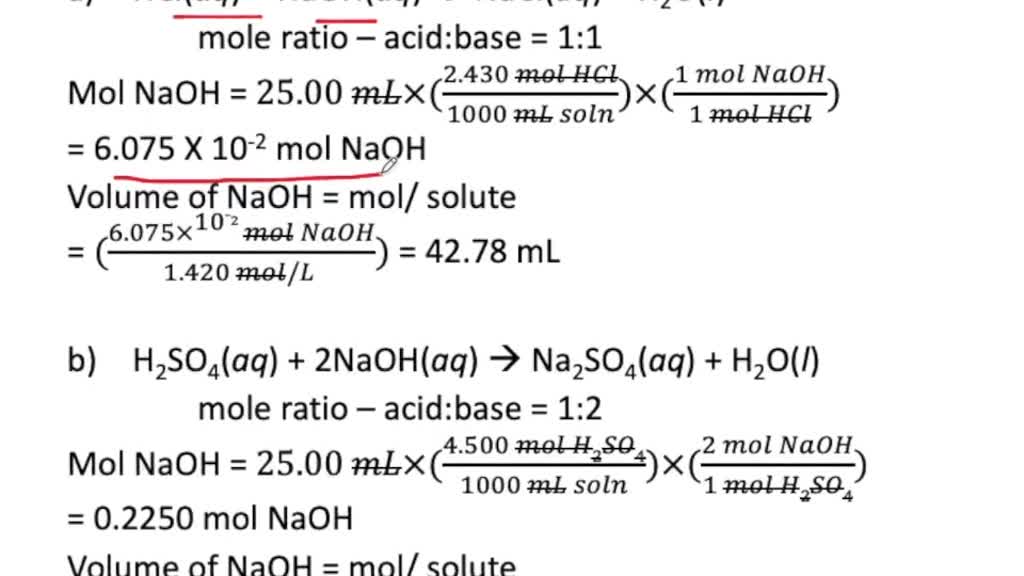
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution required to titrate the
Suppose that we now add 0.20 M NaOH to 50.0 mL of a 0.10 M solution of HCl. Because HCl is a strong acid that is completely ionized in water, the initial [H +] is 0.10 M, and the initial pH is 1.00.Adding NaOH decreases the concentration of H + because of the neutralization reaction: OH − + H + ⇌ H 2 O (in part (a) in Figure 16.5.2).Thus the pH of the solution increases gradually.
Calculate pH of 0.01M solutions of NaOH
Example #1: Calculate the pH of the solution that results when 20.0 mL of 0.600 M HCl is reacted with 20.0 mL of 0.600 M NaOH solution. Solution using moles: 1) Write the balanced, chemical equation: HCl + NaOH ---> NaCl + H 2 O. 2) From the balanced equation, determine the molar ratio of the two reactants: HCl and NaOH are the reactants.
Calculate the pH of 0.05M NaOH solution Brainly.in

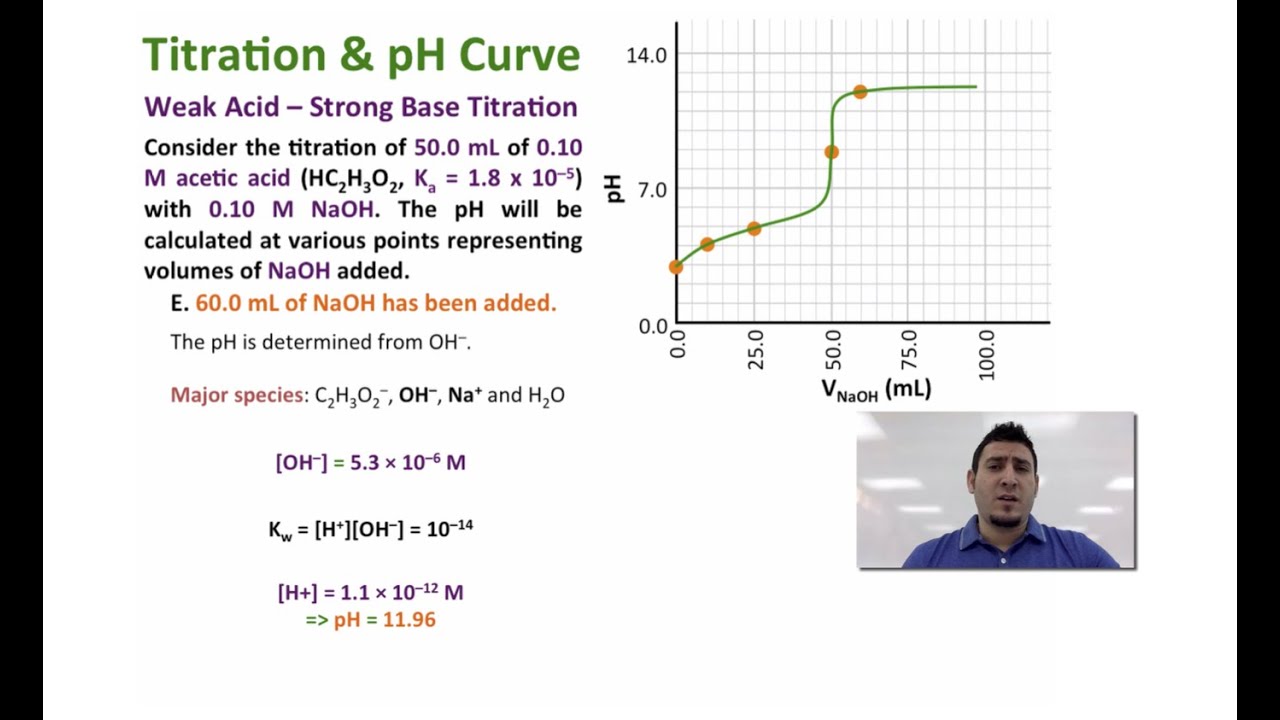
(a) As 0.200 M \(NaOH\) is slowly added to 50.0 mL of 0.100 M acetic acid, the pH increases slowly at first, then increases rapidly as the equivalence point is approached, and then again increases more slowly. The corresponding curve for the titration of 50.0 mL of 0.100 M HCl with 0.200 M \(NaOH\) is shown as a dashed line.
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision

Calculate the pH of a 0.5 M NaOH solution. There are 2 steps to solve this one. To start, calculate the pOH of the solution by applying the formula p O H = − log [ O H −], where [ O H −] represents the concentration of the hydroxide ion, which equals the molarity of NaOH in this case as it is a strong base and dissociates fully.
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH

A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH (the titration curve is shown in Figure 14.18). Calculate the pH at these volumes of added base solution: (a) 0.00 mL (b) 12.50 mL (c) 25.00 mL (d) 37.50 mL. Solution (a) Titrant volume = 0 mL. The solution pH is due to the acid ionization of.
Как определить ph раствора дома 84 фото
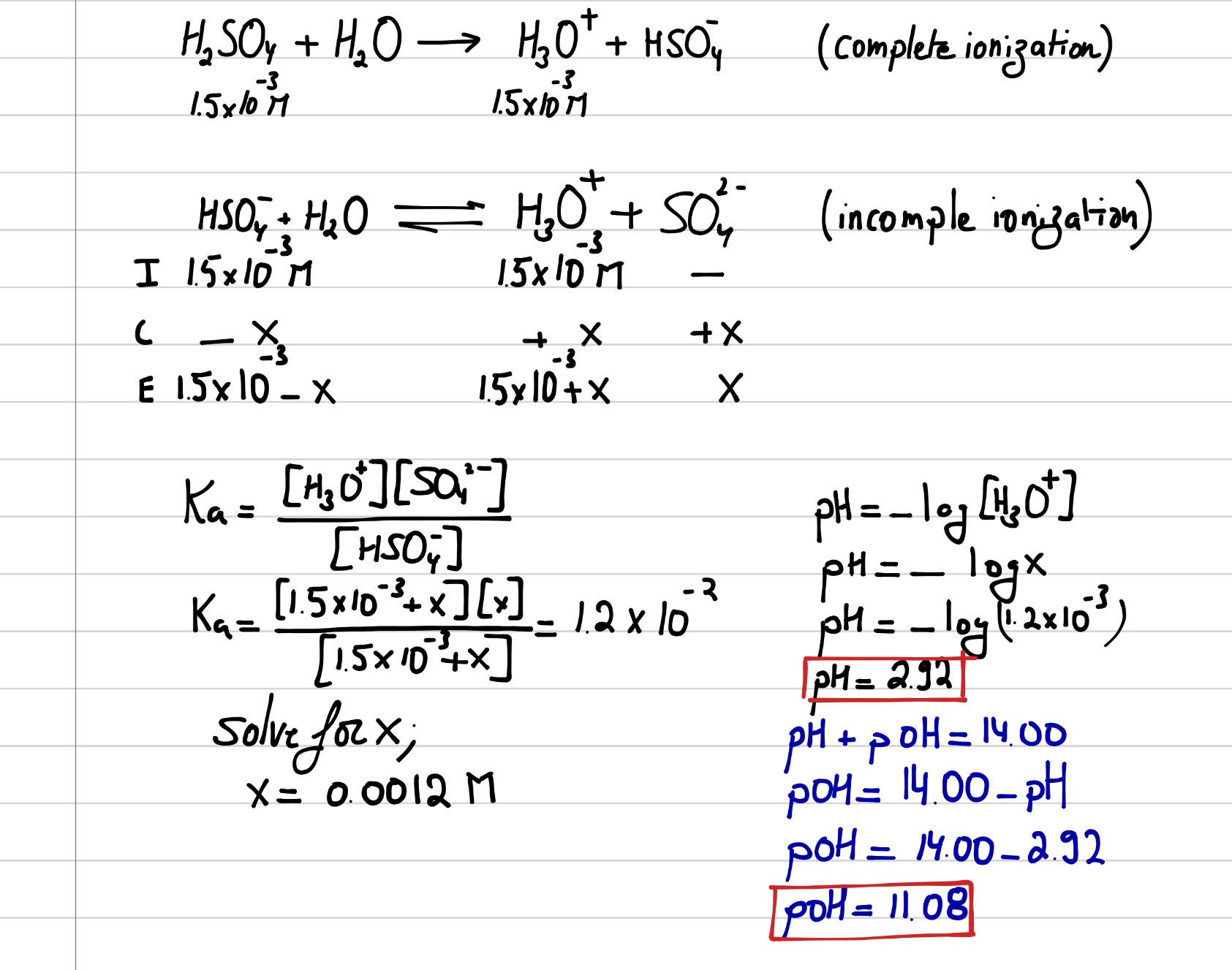
The pH is about 12.7. NaOH is a strong base, meaning it will dissociate basically completely in solution to form Na^+ and OH^- ions in solution. One mole of OH^- ions is created for every mole of NaOH that is dissolved. If it's a .05 M solution of NaOH, then it can also be interpreted to be a .05M solution of OH^-. pOH (The pH scale for bases) can be found by taking the negative logarithm of.
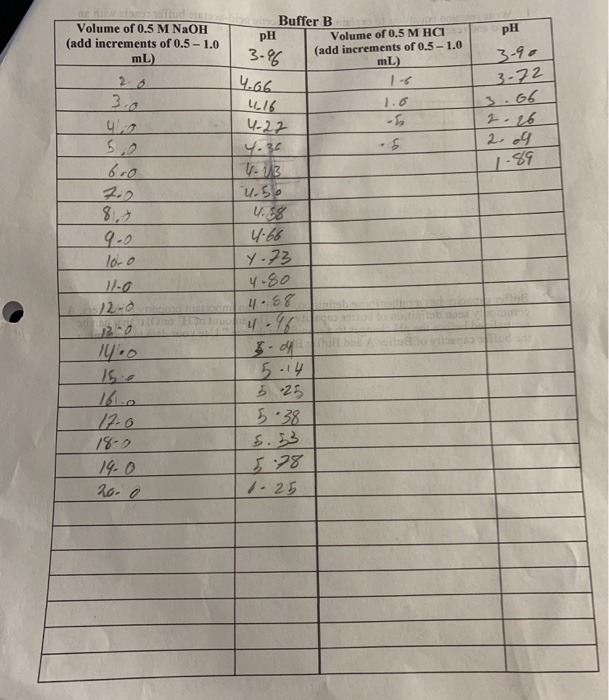
Solved pH Volume of 0.5 M NaOH (add increments of 0.5
It forms a weak basic solution. First, you should know that, is it possible to have a concentration like 0.32 M of 0.32 M Mg(OH) 2. You can calculate that from using solubility data of inorganic compounds. Is KOH a strong base. Yes. KOH is a strong base and it completely dissociates to K + and OH-ions in the water. calculate the ph of naoh
Solved 3. Calculate the pH of a 0.0010 M NaOH solution and

Multiply the molarity of the strong base NaOH by the volume of the NaOH (M B × V B = 0.500 M × 20.70 mL). Divide this answer (10.35 M × mL) by the volume of the acid HCl. What is the pH of 49 mL of 0.1 M HCl and 50 mL of 0.1M HCl solution? pH is 3.00. The number of moles of H + ions from HCl is equal to: 50.00 × 10-3 L × 0.100 M HCl = 5.
The pH of a solution obtained by mixing 100ml of 0.2 M CH3COOH with 100 ml of 0.2 M NaOH will be
Using a pH indicator strip will tell you that NaOH (sodium hydroxide) is a strong alkaline. This means it has a pH toward the top end of the pH scale, which ranges from 0 to 14. To calculate the exact pH, work out the molarity of the solution, then apply that to the formula for pH.
A Amostra De 100 Ml De Naoh EDUCA

A 100 ml $\ce{HCl}$ solution has a pH of $3.7$. You want the solution to be of pH 4.5. You have a solution of $10\ \mathrm M$ $\ce{NaOH}$. How much $\ce{NaOH}$ do you need to add to to the $100\ \m.
The Soultion of naoh contains 0.04 gm of naoh per litre. Its Ph is
Calculate the pH of 0.5 M NaOH (strong base). There are 2 steps to solve this one. Expert-verified. 100% (1 rating) Share Share.
.